Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
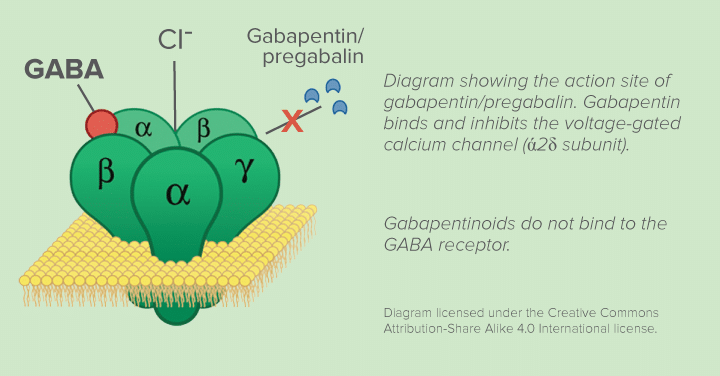
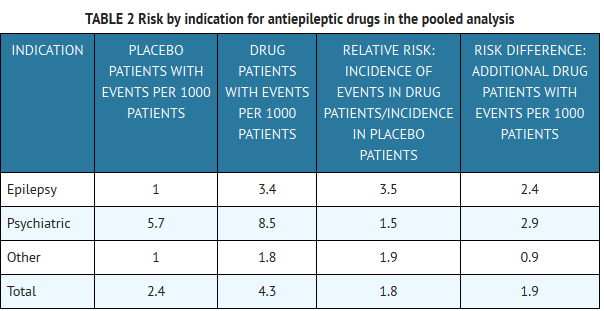
Gabapentin is a controlled substance in states like Michigan and Kentucky, while others have mandated reporting rules. Learn about its risk for abuse here. The schedule depends on the drug’s potential for abuse or dependency. Schedule V drugs have a lower potential for abuse than all other controlled substances. This means gabapentin has a lower risk of abuse compared to Oxycontin (oxycodone), which is a schedule II opioid medication. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. Corrected Classification for Schedule I Controlled Substances (12/10/2015) Placement of Eluxadoline into Schedule IV for Controlled Substances Placement of Acetyl Fentanyl into Schedule I of the S.C. Controlled Substances Act Removal of [123I]ioflupane from Schedule II of the S.C. Controlled Substances Act DEA Scheduling of Three Synthetic Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. View gabapentin information, including dose, uses, side-effects, renal impairment, pregnancy, breast feeding, monitoring requirements and important safety information. We would like to show you a description here but the site won’t allow us. 2023 South Carolina Code of Laws Title 44 - Health Chapter 53 - Poisons, Drugs And Other Controlled Substances Section 44-53-190. Schedule I. TO AMEND THE SOUTH CAROLINA CODE OF LAWS BY AMENDING SECTION 44-53-160, RELATING TO PROCESSES FOR CHANGING CONTROLLED SUBSTANCE SCHEDULES, SO AS TO REQUIRE THE STATE BOARD OF PHARMACY TO PERFORM FUNCTIONS TO QUICKLY IDENTIFY NEW SYNTHETIC CHEMICAL FORMULAS FOR SCHEDULING AND TO AUTHORIZE THE STATE BOARD OF PHARMACY TO ISSUE EMERGENCY RULES TO SCHEDULE SYNTHETIC CHEMICAL FORMULAS AS A Legislative Oversight Study of the Department of Health and Environmental Control: Department’s Response to Follow-Up Questions The South Carolina Drug Laws recognize five (5) separate and distinct categories of drugs into “Schedules.” What schedule a certain drug may fall into depends on the drug’s acceptable medical use as well as the drug’s potential for abuse or dependency. HISTORY: 1962 Code Section 32-1510.29; 1971 (57) 800. SECTION 44-53-180. Tests for inclusion of substance in Schedule I. The Department shall place a substance in Schedule I if it finds that the substance has: (a) A high potential for abuse; (b) No accepted medical use in treatment in the United States; and The list of controlled substances posted on the DPH website is for informational purposes only. The posted list includes all substances scheduled in accordance with the provisions of SC Code Section 44-53-160, designated by DPH and transmitted to the General Assembly since the last SC Code update in 2012, as well as the list of scheduled substances currently in the SC Code for Schedules I-V SOUTH CAROLINA BOARD OF MEDICAL EXAMINERS’ 2023 PAIN MANAGEMENT GUIDELINE1 The Centers for Disease Control and Prevention (CDC) has recently published the CDC Clinical Practice Guideline for Prescribing Opioids for Pain—United States, 2022 (“Guideline”). This guideline provides recommendations for clinicians providing pain care, including those prescribing opioids, for outpatients aged The physician must either hold a permanent, active, and unrestricted authorization to practice medicine in South Carolina and must be actively practicing on the ground within the geographic boundaries of South Carolina or hold an active, unrestricted academic license to practice medicine in South Carolina and must be actively practicing on the Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin is classified as a controlled substance in several states, including Alabama, Georgia, Kentucky, Tennessee, and Texas. These states have placed it under Schedule V, indicating a lower potential for abuse compared to higher schedules. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |