Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 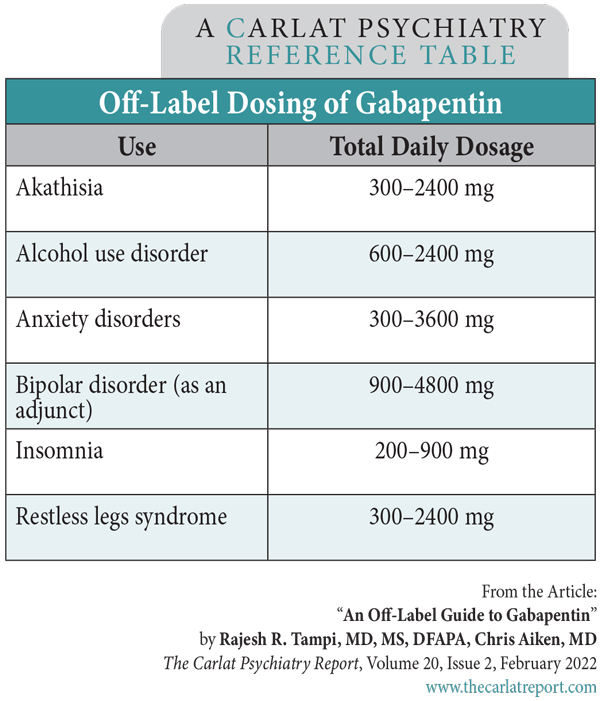 |
Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation Please be advised that the information contained in this table is compiled solely from reference works recognized and approved by the State Board of Pharmacy pursuant to rule 4729:9-2-01. A common drug sometimes prescribed as an opioid alternative is being abused across Ohio, and its misuse could lead the state to deem it a controlled substance in the near future. Gabapentin, also From the Director’s Desk Effective November 15, 2022, Ohio Administrative Code (OAC) 4729:5-5-08 was amended to require pharmacists to check a patient’s Ohio Automated Rx Reporting System (OARRS) report prior to dispensing medications containing gabapentin. Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). What is OARRS? ted Rx Reporting System (OARRS). OARRS collects information on all outpatient prescriptions for controlled substances and gabapentin dispensed by Ohio-licensed pharmacies and personally furnished OARRS also tracks naltrexone dispensed by pharmacies to Ohio patients and medical marijuana sold by Ohio dispensaries. • Data are from the Ohio Automated Rx Reporting System Prescription Drug Monitoring Program (PDMP), which collects all Schedule II, III, IV and V controlled substance prescriptions and two non - controlled drugs (gabapentin and naltrexone) dispensed by Ohio pharmacies and personally furnished by Ohio prescribers. • This presentation contains preliminary OARRS statistics or aggregate data Required OARRS Review for Gabapentin Prescriptions -Effective 11/15/22 Effective November 15, 2022, OAC 4729:5-5-08 will be amended to require pharmacists to check a patient’s OARRS report prior to dispensing medications containing gabapentin. This rule is intended to address an increase in the overutilization of gabapentin as reported by OARRS and Board staff. For example, 24.9% (159,163 From the Director’s Desk Effective November 15, 2022, Ohio Administrative Code (OAC) 4729:5-5-08 was amended to require pharmacists to check a patient’s Ohio Automated Rx Reporting System (OARRS) report prior to dispensing medications containing gabapentin. But several states consider gabapentin a schedule V (schedule 5) controlled substance. In states where gabapentin is a controlled substance, there’s stricter laws regarding prescribing and dispensing it from pharmacies. Beginning December 1, 2016, the State of Ohio Board of Pharmacy requires pharmacies, prescribers, and wholesalers to report the dispensing, personal furnishing, and wholesale sale of all products containing gabapentin (brand names: Neurontin, Gralise, Horizant) to the Ohio Automated Rx Reporting System (OARRS). We would like to show you a description here but the site won’t allow us. Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. This website publishes administrative rules on their effective dates, as designated by the adopting state agencies, colleges, and universities. Gabapentin Abuse is on the Rise: Ohio Officials Consider Making Changes Gabapentin, an opioid alternative, is being abused at an alarming rate across Ohio. As a result, state officials are considering reclassifying this drug as a controlled substance. Gabapentin (Neurontin) is typically prescribed to treat seizures and nerve pain in adults. Prescribing Controlled Substances Rules and FAQs.State Medical Board of Ohio | 30 East Broad Street, 3rd Floor, Columbus, OH 43215 | Call: 614-466-3934 Annual Review Completed for all Drug Entries on 9-15-2019 Please be advised that the information contained in this table is compiled solely from reference works recognized and approved by the State Board of Pharmacy pursuant to rule 4729-11-07. Update on Gabapentin in Ohio As a reminder, gabapentin is not considered a controlled substance in Ohio. The Board was made aware of incorrect communications made by a third-party vendor stating that Ohio had made gabapentin a controlled substance. In general, schedule II controlled substance prescriptions cannot be faxed. Exceptions to this are outlined in rule 4729:5-3-11 of the Ohio Administrative Code.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |