Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
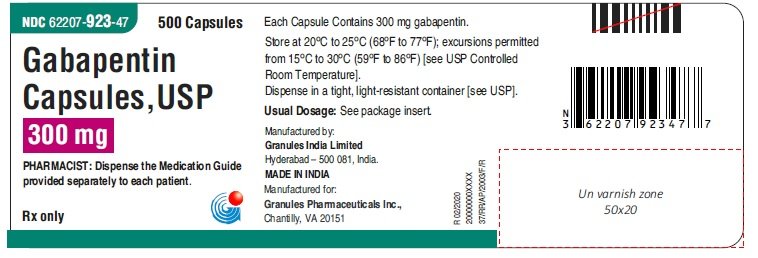 |  |
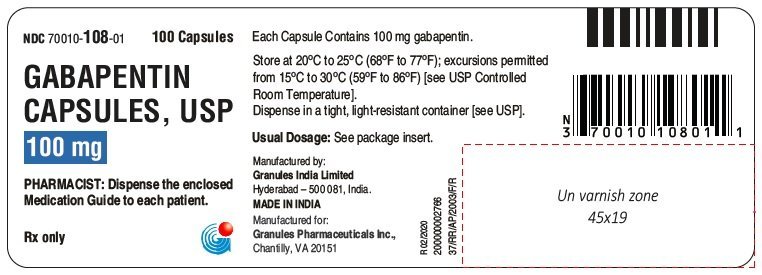
In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). HORIZANT provides approximately dose-proportional and extended exposure to 346 gabapentin over the range 300 to 6,000 mg. HORIZANT and gabapentin are not interchangeable 348 347 because the same daily dose of each results in different plasma concentrations of gabapentin. 349 Absorption: The pathway for absorption of gabapentin enacarbil is Patients taking gabapentin should not drive until they have gained sufficient experience to assess whether gabapentin impairs their ability to drive. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended release) indicate that gabapentin may cause significant driving impairment. Advise the patient to read the FDA-approved patient labeling (Medication Guide). Administration Information - Inform patients that gabapentin capsules are taken orally with or without A literature article reported that when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg NEURONTIN capsule (N=12), mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. Important Limitation: GRALISE is not interchangeable with other gabapentin products because of differing pharmacokinetic profiles that affect the frequency of administration (See Warnings and Precautions) DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The Center for Drug Evaluation and Research (CDER) ensures that safe and effective drugs are available to improve the health of the people in the United States DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. HORIZANT provides approximately dose-proportional and extended exposure to gabapentin over the range 300 to 6,000 mg. HORIZANT and gabapentin are not interchangeable because the same daily dose of each results in different plasma concentrations of gabapentin. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more Gabapentin is most commonly used in veterinary medicine to relieve chronic pain and in some pets, to reduce fear and anxiety associated with veterinary appointments. Gabapentin may be used alone or in combination with other drugs. Gabapentin is available as capsules, tablets and as an oral solution. The starting dose is 300 mg three times a day. The recommended maintenance dose of gabapentin Capsules is 300 mg to 600 mg three times a day. Dosages up to 2400 mg/day have been administered in long-term clinical studies. Doses of 3600 mg/day have also been administered to a small number of patients for a relatively short duration. Administer gabapentin capsules three times a day using 300 mg Drugs @ FDA 8 , where information about FDA-approved human brand name and generic drugs as well as therapeutic biological products is available. Biologics Products and Establishments 9 , where information about vaccines, allergenics, and blood products is available. Office of Nonprescription Products 10 Guidance on Gabapentin This guidance represents the Food and Drug Administration's (FDA's) current thinking on this topic. Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR)
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |